
MAGNESIUM SULPHATE FOR FETAL NEUROPROTECTION TO PREVENT CEREBRAL PALSY (MAG-CP)
The most recent project of the Canadian Perinatal Network (CPN) was MAG-CP (2011-2016). MAG-CP used the existing standardized national database of pregnancies at high risk of very preterm birth at 22+0 to 28+6 weeks gestation, which was created as part of the BILBO project, to audit magnesium sulphate (MgSO4) usage in threatened preterm birth and preterm birth, and monitor maternal and perinatal outcomes.
Preterm birth is the leading cause of infant death, illness, and disability in Canada and worldwide. Despite marked improvements in survival rates of preterm infants, the risk of neurodevelopmental impairment including cerebral palsy (CP), is substantial and is not improving. The overall prevalence of CP is 2-2.5 per 1000 live births, but the risk is up to 80-fold higher for babies born at <28 weeks. MgSO4 for fetal neuroprotection provides one of the first prenatal evidence-based treatments to improve the neurodevelopmental outcomes of children born preterm. The Society of Obstetricians and Gynaecologists of Canada (SOGC) published guidelines recommending use of MgSO4 for fetal neuroprotection in the setting of imminent preterm birth at <32 weeks.
Although MgSO4 is inexpensive and used routinely in Canada for eclampsia prophylaxis, ongoing controversies prevent implementation and administration of MgSO4 into clinical practice, including the effect on newborn respiratory drive and the potential for increased neonatal resuscitation requirements, inadequately studied outcomes (such as overall functioning beyond 2 years of age), and a lack of understanding about the mechanism of action of MgSO4.
As such, the overall goal of the MAG-CP project was to actively operationalize the national implementation of MgSO4 for neuroprotection into clinical practice (i.e., facilitated knowledge translation). Our specific aims were to determine, for pregnancies at risk of preterm birth at <29 weeks, whether maternal administration of MgSO4 for fetal neuroprotection:
- Could be increased to a pre-specified level of 80% of eligible women over 4 years;
- Is associated with the anticipated decrease in CP or ‘death or CP’ that was seen in the RCTs; and
- Is associated with an acceptable safety profile for mothers and babies.
GENERAL HYPOTHESIS
In the setting of very preterm birth, we anticipated that the timing and degree of uptake of MgSO4 utilization would vary between hospitals. We anticipated a decrease in both infant outcomes of interest: CP and ‘death or CP’. Additionally, we anticipated an increase in minor maternal side effects, but no increase in neonatal resuscitation, and no increase or decrease in stillbirth or neonatal death is anticipated.
METHODS
MAG-CP focused on babies at risk of being born at <29 weeks, who were those identified by the CPN which focused on threatened very preterm birth at <29 weeks. CPN links to the Canadian Neonatal Follow-Up Network (CNFUN) that tracks these babies’ neurodevelopment to 3 years of age. In participating tertiary perinatal centres, we used the seven-phase KT translation cycle, which includes an exploration of barriers to, and facilitators of, practice change within each site.
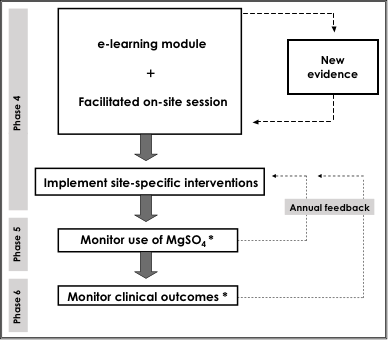
OUTCOMES
- The primary outcome were the rates of MgSO4 utilization.
- Our secondary outcome was CP, or ‘death or CP’. Our other outcomes included maternal side effects, and neonatal adverse effects, including resuscitation.
IMPACT
For babies at risk of being born at <29 weeks, we have an internationally unique opportunity to translate MgSO4 for fetal neuroprotection into practice, monitor MgSO4 use and outcomes, and settle controversies.
PUBLICATIONS
- De Silva DA, Synnes AR, von Dadelszen P, Lee T, Bone JN, Magee LA. MAGnesium sulphate for fetal neuroprotection to prevent Cerebral Palsy (MAG-CP)—implementation of a national guideline in Canada. Implementation Science. 2018 Dec;13(1):8.
- Teela KC, De Silva DA, Chapman K, Synnes AR, Sawchuck D, Basso M, Liston RM, von Dadelszen P, Magee LA. Magnesium sulphate for fetal neuroprotection: benefits and challenges of a systematic knowledge translation project in Canada. BMC Pregnancy Childbirth. 2015 Dec 22;15(1):1
- De Silva DA, Sawchuck D, von Dadelszen P, Basso M, Synnes AR, Liston RM, Magee LA on behalf of the MAG-CP Collaborative Group. Magnesium sulphate for eclampsia and fetal neuroprotection: a comparative analysis of protocols across Canadian tertiary perinatal centres. J Obstet Gynaecol Can. 2015;37(11):975-87.
- Nensi A, De Silva DA, von Dadelszen P, Sawchuck D, Synnes AR, Crane J, Magee LA on behalf of the MAG-CP Collaborative Group. Effect of magnesium sulphate on fetal heart rate parameters: a systematic review. J Obstet Gynaecol Can. 2014;36(12):1055-64.
- Bickford CB, Magee LA, Mitton C, Kruse M, Synnes AR, Sawchuck D, Basso M, Senikas VM, von Dadelszen P, on behalf of the MAG-CP Working Group. Magnesium sulphate for fetal neuroprotection: a cost-effectiveness analysis. BMC Health Serv Res. 2013;13:527.
- Magee L, Sawchuck D, Synnes A, von Dadelszen P. SOGC Clinical Practice Guideline. Magnesium sulphate for fetal neuroprotection. J Obstet Gynaecol Can. 2011 May;33(5):516-29.
FUNDER
- Bill & Melinda Gates Foundation (through PRE-EMPT)
PARTNERS
- Maharashtra, India
- Co-ordinating Centre: Gynuity Health Project