Development and Validation of the PIERS (Pre-eclampsia Integrated Estimate of RiSk) models

BACKGROUND
This project continues a line of enquiry by the PIERS research group to develop and validate outcome prediction models for use to aid clinical decision making around care of women with pre-eclampsia. The initial model developed by this research group, the fullPIERS model, is based on a combination of maternal symptoms, signs and laboratory variables. This model accurately identifies women at incremental risk of maternal complications of pre-eclampsia (area under the receiver operator curve [AUC ROC]: 0.88 to predict adverse maternal outcomes within 48h). The fullPIERS model was developed and validated in eight tertiary academic centers in Canada, UK, NZ, and Australia. More details on this model are available here.
Although the immediate relevance of the fullPIERS model pertains to high-income tertiary care centres, potential research and implementation applications span prevention, early detection, community-based stabilisation, and facility-based management of pre-eclampsia. Moreover, a clear research and implementation priority is to adapt and test the PIERS model for low-income and middle-income settings, especially in south Asia and sub-Saharan Africa. In LMICs, where laboratory tests are not always readily available, the fullPIERS model may not be practical or cost effective. A simplified version of this model for use in community and PHC facilities is needed.
MINIPIERS OVERVIEW
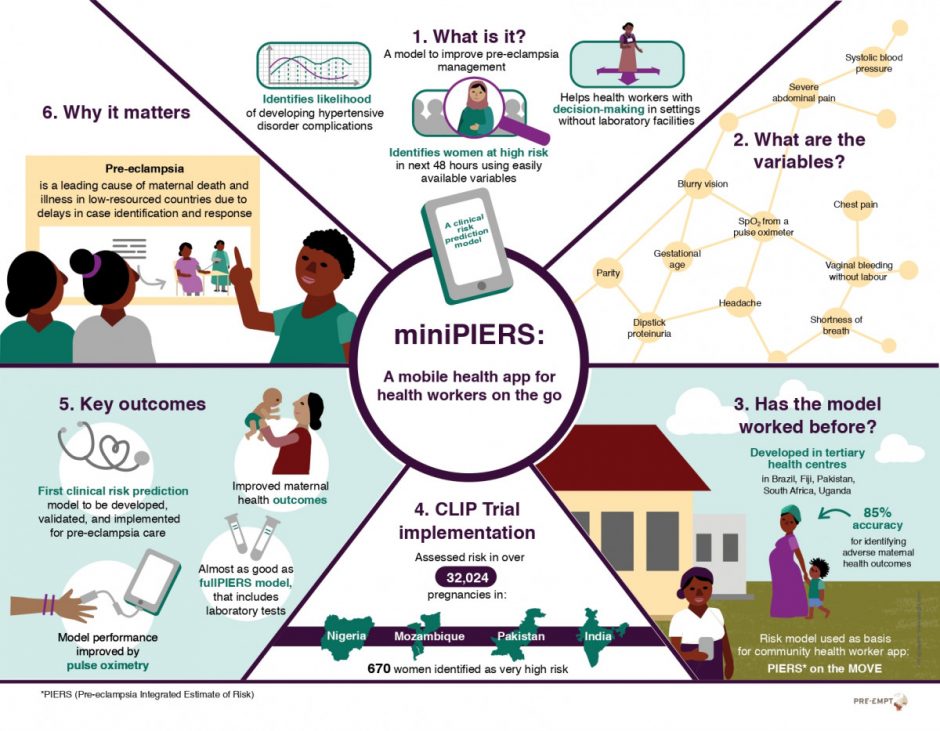
The hypertensive disorders of pregnancy (HDP) are one of the leading causes of maternal death and morbidity in low-resourced countries due to delays in case identification and a shortage of health workers trained to manage the disorder. The objective of the miniPIERS study was to develop an evidence-based tool that can aid community-based health workers in decision making around the care of women with hypertensive disorders of pregnancy.
The miniPIERS project concluded its active phase of research in 2012, which resulted in the following manuscript.
- Payne BA, Hutcheon JA, Ansermino JM, Hall DR, Bhutta ZA, Bhutta SZ, Biryabarema C, Grobman WA, Groen H, Haniff F, Li J. A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low-resourced settings: the miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) multi-country prospective cohort study. PLoS medicine. 2014 Jan 21;11(1):e1001589.
Since then the focus has been on secondary analysis of the valuable dataset this study provided. In 2015, we were able to meet the objectives set out in the 2013-2014 annual report and have completed the following manuscripts based on miniPIERS data.
- Payne BA, Hutcheon JA, Dunsmuir D, Cloete G, Dumont G, Hall D, Lim J, Magee LA, Sikandar R, Qureshi R, van Papendorp E. Assessing the Incremental Value of Blood Oxygen Saturation (SpO 2) in the miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) Risk Prediction Model. Journal of Obstetrics and Gynaecology Canada. 2015 Jan 31;37(1):16-24.
- Payne BA, Groen H, Ukah UV, Ansermino JM, Bhutta Z, Grobman W, Hall DR, Hutcheon JA, Magee LA, von Dadelszen P, miniPIERS working group. Development and internal validation of a multivariable model to predict perinatal death in pregnancy hypertension. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2015 Oct 31;5(4):315-21.
The miniPIERS model provided the foundation for development of the decision algorithm used in the CLIP trial as part of the PIERS On the Move (POM) tool (more details on POM here). In 2015, the CLIP Pilot Trials in Nigeria, Pakistan and India concluded. A primary focus of the Pilot trials was to determine the feasibility of the PIERS On the Move tool in a real clinical setting. Lessons learned during this phase of study led to updates of the POM app used in each country during the Definitive phase of the CLIP study.
NEXT STEPS
Future goals include: develop methodology for updating the prediction model after clinical implementation and to use these new methods to update the miniPIERS model as needed.
Post-trial evaluation of the impact of POM on the health workers’ knowledge, skills and perception of value as a result of using the app.